在当前微流控技术发展中,芯片上的细胞培养、活细胞成像、3D细胞培养及药物毒性测试等实验越来越受到广大的高校、研究所及企业研究人员的关注。微流控技术在上述应用领域提供了比传统实验室无可比拟的优势比如实验耗材量小、实验速度快、占用空间小、费用低等。针对上述的应用,本文介绍一套微流控细胞灌注实验系统,凭借该套系统,您可以放心的做芯片上的细胞培养、活细胞成像、3D细胞培养、药物筛选等实验。


微流控细胞灌注套装(适用于微流体芯片和灌注实验)的优势:
(1)无脉冲
实验过程中完全稳定的流动
(2)受控剪切应力
通过各种流量控制剪切应力
(3)方便易用
实验所需组件全部在放在一个套装里面包括软件

一种致力于细胞培养的流体系统
Elveflow提供的微流控细胞灌注系统可专门用于细胞培养、实验室芯片和灌注室(perfusion chambers)实验。这种完全集成的解决方案包括创建连续流量和监视施加在样品池上流体流量所需的全部实验组件。
可在达10种不同的培养基或试剂之间进行切换
对于需要在不同培养基或药物之间切换的实验来说,计算机控制的微流体阀允许您按照一定的次序进行注射。
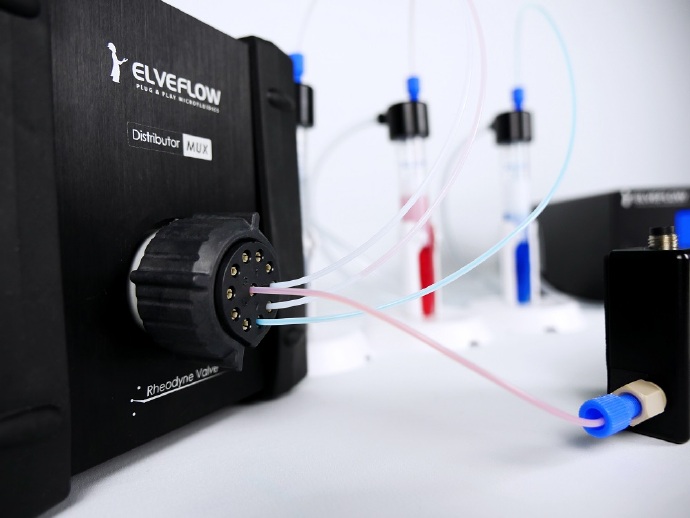
微流控细胞灌注系统的特点&优势
实验原理
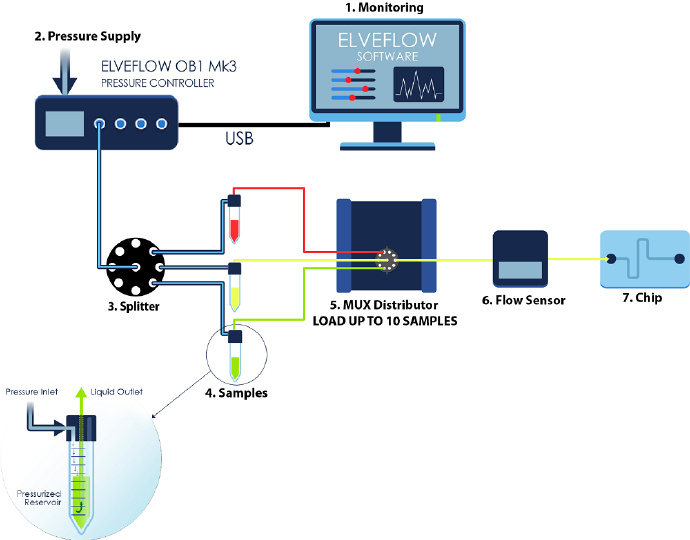
(1)控制压力和流速
非常适合剪切应力测定
(2)不同的介质或药物之间进行快速切换
用于成像细胞对各种介质或药物的反应
(3)稳定且无脉冲流量
没有更多的盖玻片扩张和细胞压力
(4)流量范围广
从10 nL/min到5 mL/min
(5)设计流动注射序列
创建复杂的流动模式例如模拟生理条件的振荡流
(6)循环回路
非常适合长期检测的实验
(7)瞬间停止流动
用于受控溶液暴露实验例如钙成像
微流控细胞灌注系统的组件
(1)OB1 MK3+压力&流量控制器:施加给定压力以产生稳定且无脉冲的流速。
(2)样品储液池:放置您的培养基或样品。从Eppendorf到瓶子都有各种尺寸可供选择。
(3)旋转阀:选择注入的液体。
(4)流量传感器MFS或BFS:实时监控流量。
(5)灌注室或微流体芯片:放置您实验用的细胞。与显微镜相兼容。
(6)计算机/电脑:使用我们的智能界面软件ESI来控制实验的所有参数,并通过创建注射序列来自动化的进行您的实验。
(7)微流控导管和连接器:用于微流控器件和芯片之间的相互连接。
微流控细胞灌注系统的应用
(1)芯片上的细胞培养
(2)活细胞成像
(3)细胞对介质变化的反应
(4)药物筛选
(5)毒性测试
(6)干细胞分析
(7)钙成像
(8)3D细胞培养
(9)生物反应器研究
参考文献
Alexandre Grassart, ValérieMalardé,
Samy Gobaa, Anna Sartori-Rupp, Jordan Kerns, Katia Karalis, Benoit
Marteyn, Philippe Sansonetti, Nathalie Sauvonnet, Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection, Cell Host & Microbe, 2019, 26(3): 435 - 444. doi.org/10.1016/j.chom.2019.08.007
实验室内的微流控3D细胞培养系统装置设置
其他相关参考文献
● Critical Frequency and Critical Stretching Rate for Reorientation of Cells on a Cyclically Stretched Polymer in a Microfluidic Chip, Jiandong Ding et al., ACS Applied Materials & Interfaces (2021). DOI: 10.1021/acsami.0c21186
● In vitro skin model for characterization of sunscreen substantivity upon perspiration, Fatemeh Keshavarzi et al., International Journal of Cosmetic Science (2021). DOI: 10.1111/ics.12703
● Electrokinetic sandwich assay and DNA mediated charge amplification for enhanced sensitivity and specificity, Siddharth Sourabh Sahu et al. Biosensors & Bioelectronics (2021). DOI: 10.1016/j.bios.2020.112917
● The method to dynamically screen and print single cells using microfluidics with pneumatic microvalves, Chang Chen et al., MethodX (2020). DOI: 10.1016/j.mex.2020.101190
● Cyclic on-chip bacteria separation and preconcentration, Vitaly Ryzhkov et al., Scientific Reports (2020). DOI: 1038/s41598-020-78298-y
● Synthetic Biology Bicistronic Designs Support Gene Expression Equally Well in vitro and in vivo, Owen Koucky et al., American Journal of Undergraduate Research (2020). DOI: 10.33697/ajur.2020.012
● Zhu Z., Geng Y., Wang Y. (2021) Monitoring Single S. cerevisiae Cells with Multifrequency Electrical Impedance Spectroscopy in an Electrode-Integrated Microfluidic Device. In: Marchisio M.A. (eds) Computational Methods in Synthetic Biology. Methods in Molecular Biology, vol 2189. Humana, New York, NY. DOI: 10.1007/978-1-0716-0822-7_9
● Investigating the Interaction Between Circulating Tumor Cells and Local Hydrodynamics via Experiment and Simulations
Pepona, M., Balogh, P., Puleri, D.F. et al. . Cel. Mol. Bioeng. (2020). DOI: 10.1007/s12195-020-00656-7
● A drug-compatible and temperature-controlled microfluidic device for live-cell imaging, Open Biology; Jul, 2016; T. Chen et al; DOI : 10.1098/rsob.160156
● A microfluidic gradient generator to simulate the oxygen microenvironment in cancer cell culture; Microelectronic Engineering; Aug, 2018; Louise Orcheston-Findlay et el; DOI : 10.1016/j.mee.2018.04.011
● Geometric Friction Directs Cell Migration; Physical Review Letters; Jul, 2013; Le Berre et al; DOI : 10.1103/PhysRevLett.111.198101
● An Integrated Microfluidic Chip and Its Clinical Application for Circulating Tumor Cell Isolation and Single‐Cell Analysis; Cytometry; Oct, 2019; Mingxin Xu et al; DOI : 10.1002/cyto.a.23902
● Mitotic Rounding Alters Cell Geometry to Ensure Efficient Bipolar Spindle Formation; Developmental Cell; Apr, 2013; Oscar M. Lancaster et al; DOI : 10.1016/j.devcel.2013.03.014
网络研讨会:自动微流体顺序和定量注入以及规模化解决方案,点击 here
微流体灌注系统的自动化运行的多个解决方案
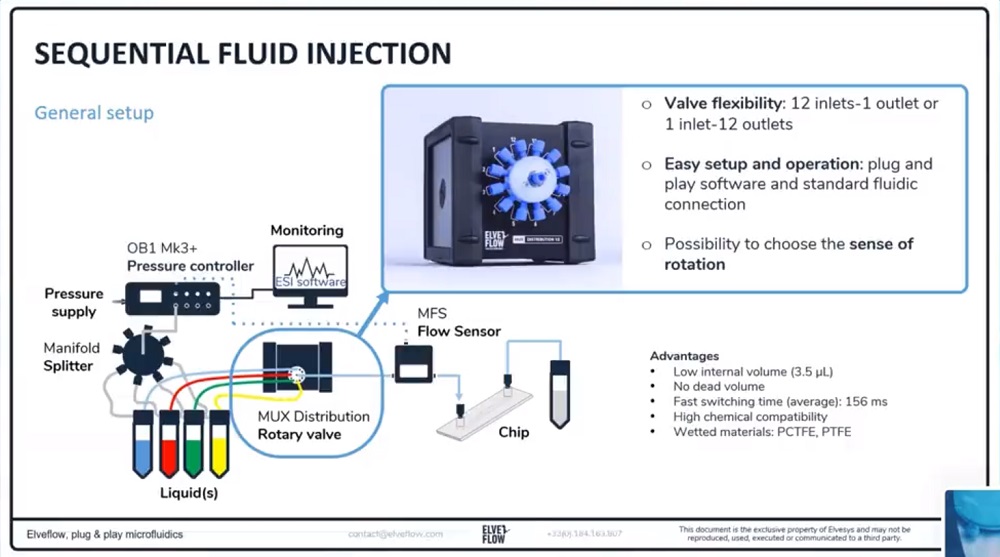
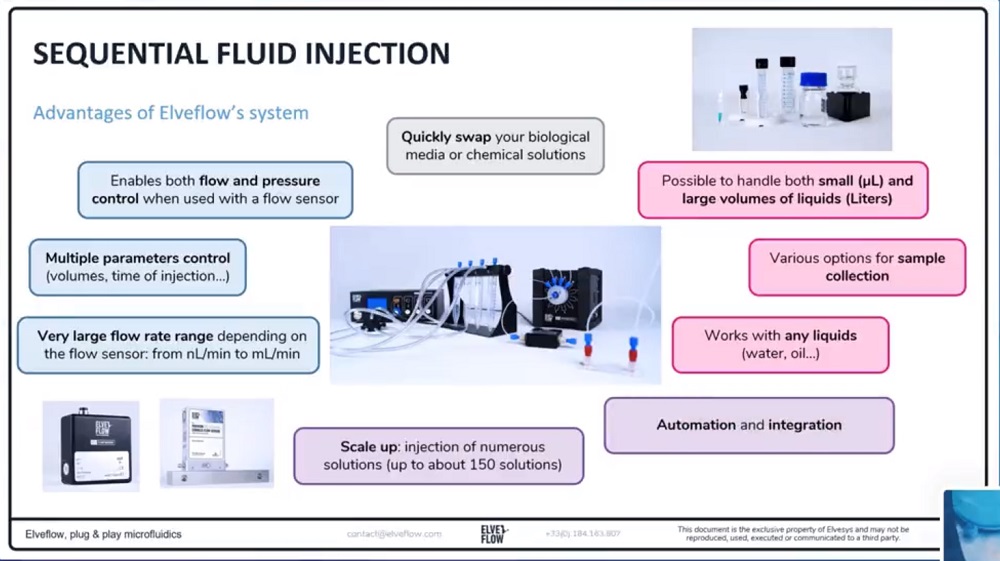
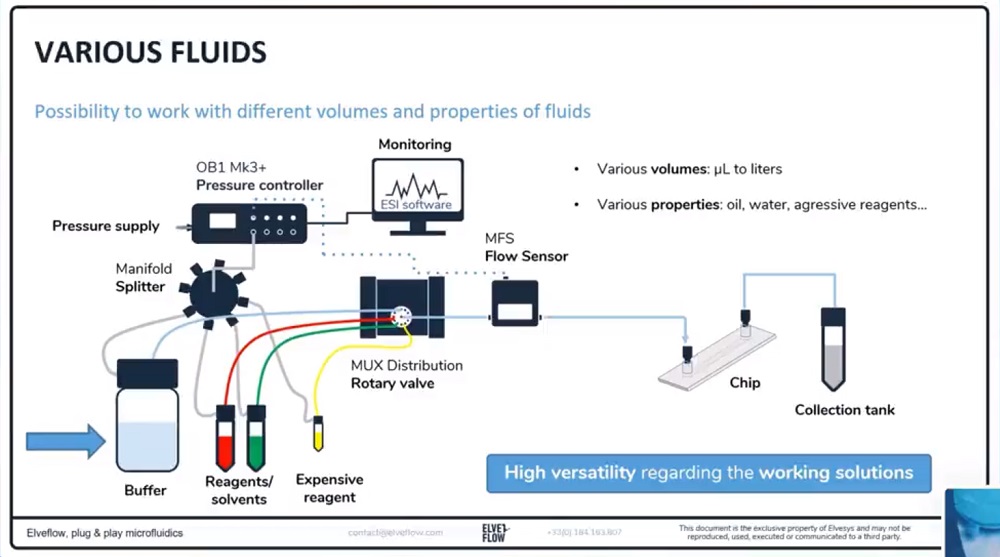
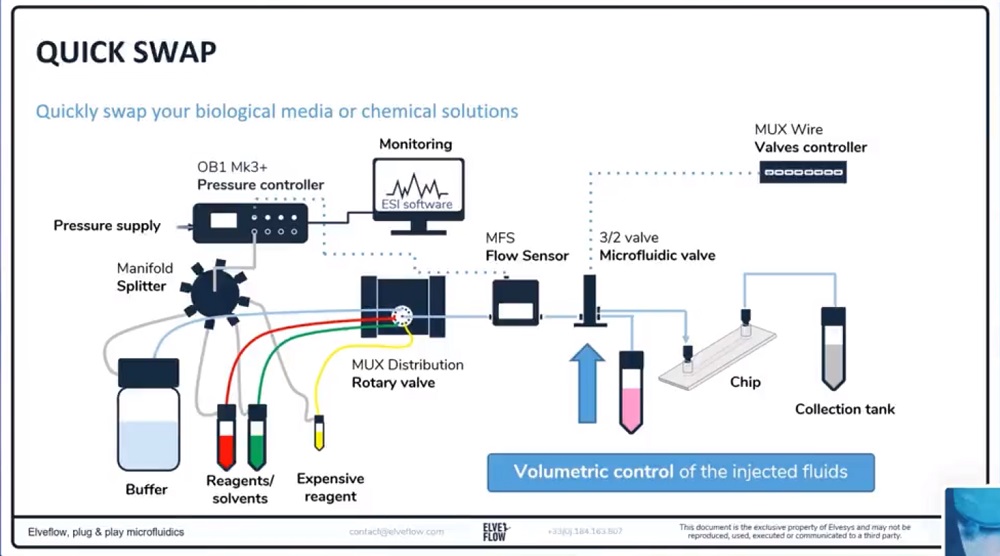
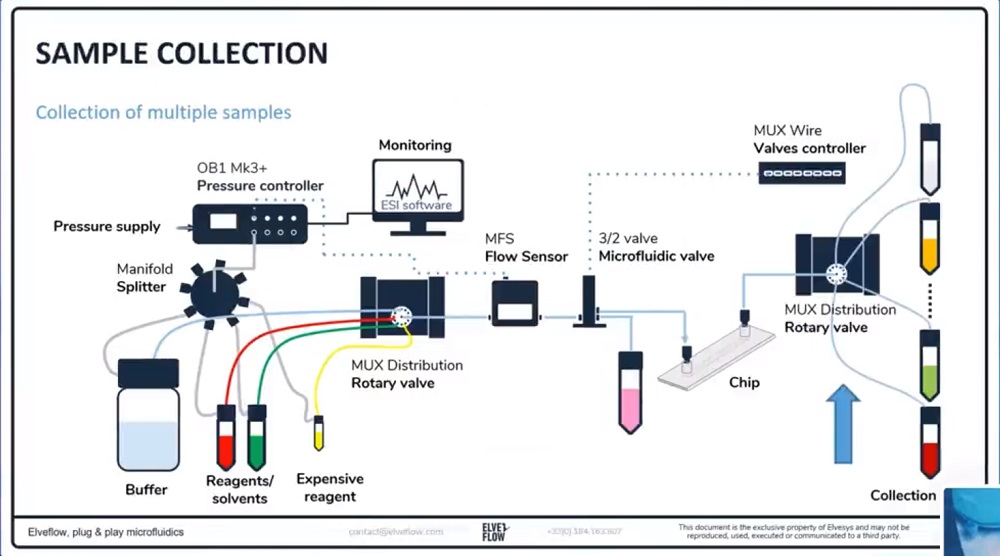
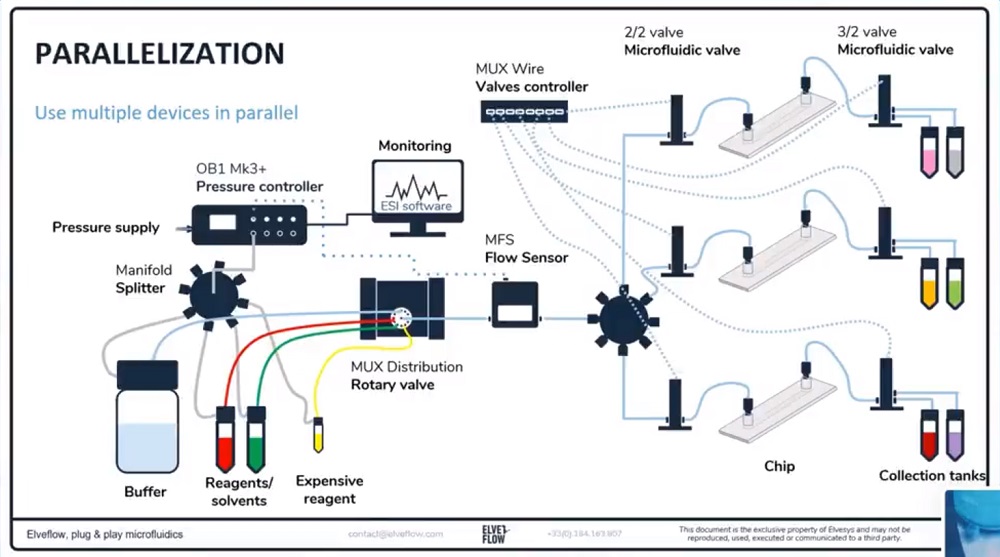
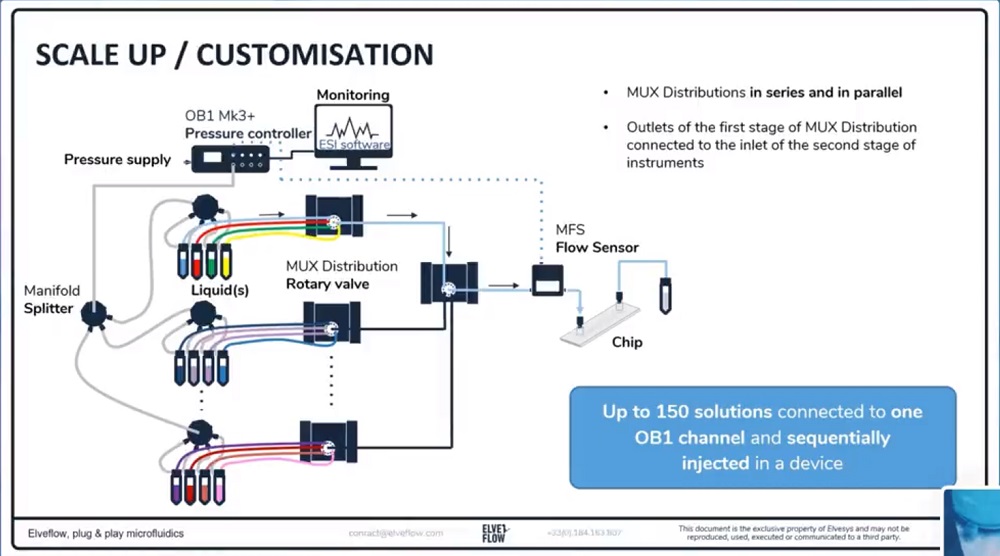
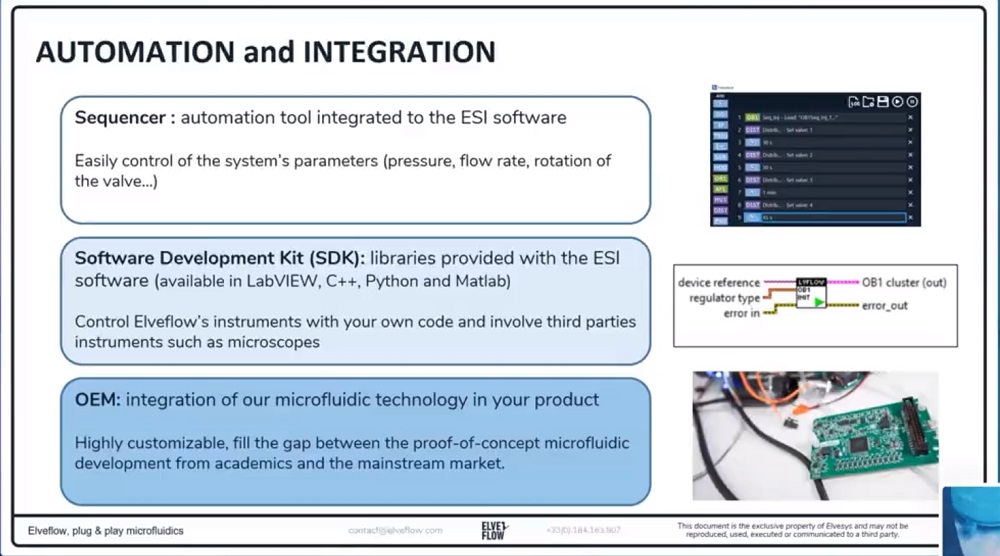
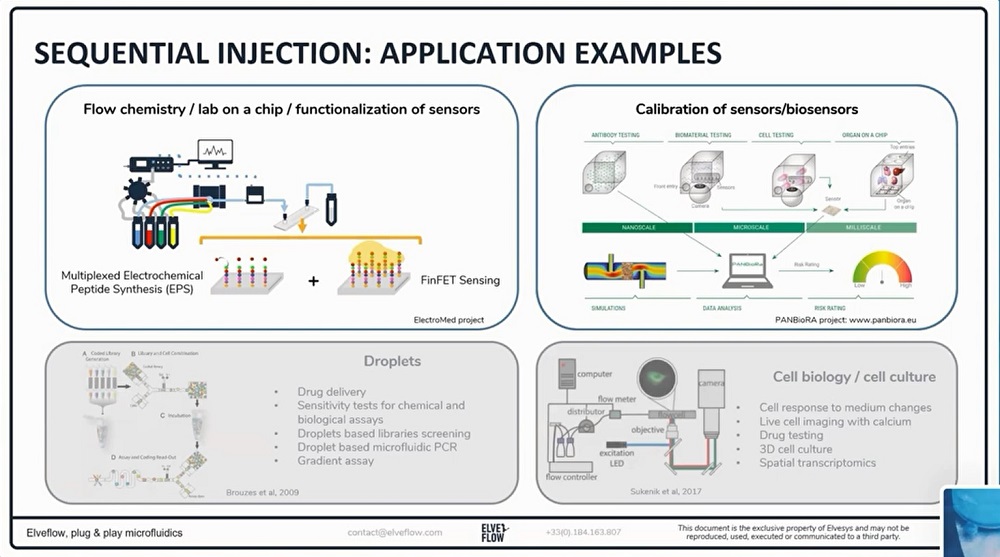
参考文献
Grassart A, Malardé V, Gobaa S, Sartori-Rupp A, Kerns J, Karalis K, Marteyn B, Sansonetti P, Sauvonnet N. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host Microbe. 2019 Oct 9;26(4):565. doi: 10.1016/j.chom.2019.09.007. Erratum for: Cell Host Microbe. 2019 Sep 11;26(3):435-444.e4. PMID: 31600505.
Tongcheng Qian, Daniel A. Gil, Emmanuel Contreras Guzman, Benjamin D. Gastfriend, Kelsey E. Tweed, Sean P. Palecekc and Melissa C. Skala, Adaptable pulsatile flow generated from stem cell-derived cardiomyocytes using quantitative imaging-based signal transduction, Lab Chip, 2020,20, 3744-3756. https://doi.org/10.1039/D0LC00546K.
Ginga NJ, Slyman R, Kim GA, Parigoris E, Huang S, Yadagiri VK, Young VB, Spence JR, Takayama S. Perfusion System for Modification of Luminal Contents of Human Intestinal Organoids and Realtime Imaging Analysis of Microbial Populations. Micromachines (Basel). 2022 Jan 14;13(1):131. doi: 10.3390/mi13010131. PMID: 35056297; PMCID: PMC8779378.
Prontera, Carmela Tania, Elisa Sciurti, Chiara De Pascali, Lucia Giampetruzzi, Francesco Biscaglia, Laura Blasi, Vanessa Esposito, Flavio Casino, Pietro Aleardo Siciliano, and Luca Nunzio Francioso. 2023. "Anodic Stripping Voltammetric Determination of Copper Ions in Cell Culture Media: From Transwell® to Organ-on-Chip Systems" Chemosensors 11, no. 8: 466. https://doi.org/10.3390/chemosensors11080466
Chun-Dong Xue, Yong-Jiang Li, Jing-Tong Na, Yan-Xia Wang, Hong-Jian Yu, Bo Liu, Tun Cao and Kai-Rong Qin, A microfluidic platform enabling real-time control of dynamic biochemical stimuli to biological cells, Journal of Micromechanics and Microengineering, 2020, 30: 095011. DOI 10.1088/1361-6439/ab9e.
Lucia Giampetruzzi, Laura Blasi, Amilcare Barca, Elisa Sciurti, Tiziano Verri, Flavio Casino, Pietro Siciliano, Luca Francioso, Advances in Trans-Epithelial Electrical Resistance (TEER) monitoring integration in an Intestinal Barrier-on-Chip (IBoC) platform with microbubbles-tolerant analytical method, Sensing and Bio-Sensing Research, Volume 37, August 2022, 100512,
https://doi.org/10.1016/j.sbsr.2022.100512.
相关应用介绍
微流控器官培养系统介绍,请点击 这里
微流控用于活细胞成像的细胞培养介绍,请点击 这里
用于药物开发的体外免疫器官芯片的介绍,请点击 这里
微流控心肌细胞培养模型(OB1压力控制器参考设置)的介绍,请点击 这里














