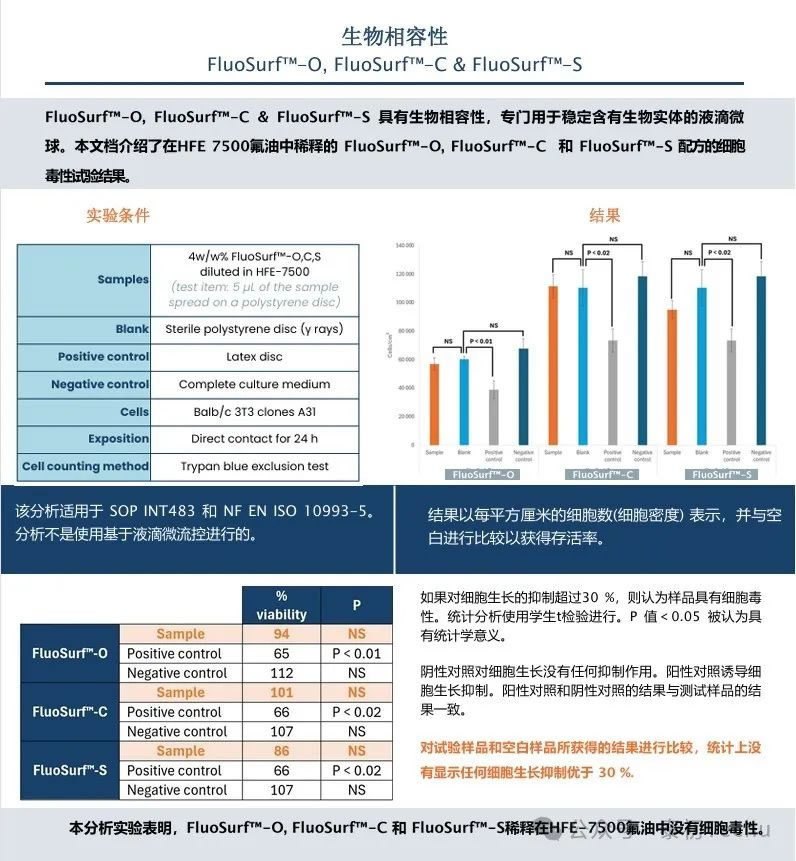
Droplet microfluidics has become a fast, accurate, quantitative, and low-cost tool for biological and chemical screening. Droplet microfluidic devices produce monodisperse water-in-oil droplets loaded with biological or chemical agents. The droplets are used as microchambers or reaction chambers. To analyze and/or classify the contents of droplets, dyes are often added. The droplets are then monitored and sorted according to their fluorescence signals.
A common problem in droplet microfluidic experiments involving dye use is dye leakage. The dye is released into the oil phase and adjacent droplets, resulting in reduced accuracy of fluorescence monitoring. Some of the parameters that affect the retention of dyes in droplets are: the nature of the buffer or cell culture medium, the choice of fluorophores and their hydrophilicity, the concentration, properties and molecular weight of the oil phase and surfactant.
In this study, we comprehensively investigated the effects of fluorine oil selection and surfactant concentration on dye leakage in microfluidic experiments. The effects of two fluorine oil and surfactant concentrations on dye retention are discussed.
I) Materials and Methods
1. Properties of Fluorinated Oils
In this study, we selected two fluorinated oils, Fluo-Oil 40 and Fluo-Oil 200, to investigate dye leakage based on microfluidic experiments. The formulations of these two fluorinated oils ensure the stability, biocompatibility, and reproducibility of microfluidic experiments, serving as alternatives to Fluorinert FC40 fluorinated oil.
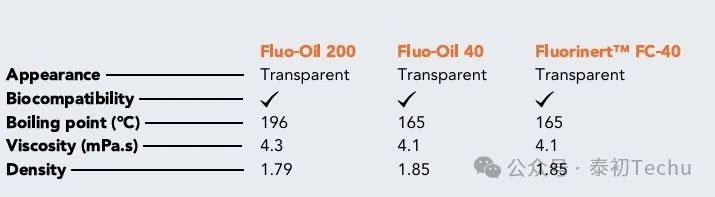
Table 1 gives the physical properties of the two fluorinated oils. The performance of the two fluorinated oils was compared with that of Fluorinert FC-40. Fluorinert FC-40 has the same performance as Fluo-Oil 40. Fluo-Oil 200 has a slightly lower density than Fluo-Oil 40 and Fluorinert FC-40, while its boiling point and viscosity are slightly higher. During the formation of droplet microspheres, it may be necessary to adjust the flow rate of the liquid slightly to obtain similar droplets, but the physical properties of the two oils are still very close.
2. Microfluidic experiments
To evaluate the effect of fluorine oil selection on dye leakage, two oils were used to generate water-in-oil droplet microspheres. Evaluate droplet size distribution, stability after incubation at 37°C, and molecular retention.
用于产生液滴微球的PDMS/玻璃微流控芯片由流动聚焦几何结构组成。微通道经过疏水试剂Fluo-ST2(疏水表面处理)处理。在比较两种氟油的研究中,通过在Fluo-Oil 200或Fluo-Oil 40中溶解4w/w%的纯FluoSurf?-C表面活性剂来制备油连续相。这些实验使用的分散水相为PBS, 20μM荧光素PBS, 2μM再间萘酚PBS或44μM resazurin + 40 mM半胱氨酸PBS。通过分析空液滴和含有荧光染料的液滴的混合物,对两种氟油进行了比较。采用注射泵控制微流控液滴芯片中不同相的流速(油相:300μL/h,水相:100μL/h)进行液滴稳定性实验;油相:600μL/h,水相:100μL/h进行染料保留实验)。
Monitor droplet generation with an inverted microscope. Image analysis was performed using Image J software.
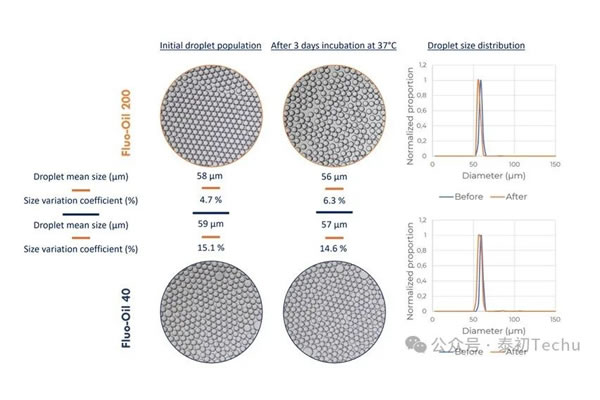
II)孵育后液滴稳定性
在两种氟油(Fluo-Oil 40和Fluo-Oil 200)中通过孵育研究了液滴的稳定性。在37°C孵育3天前后分析液滴的大小分布。
The results are shown in Figure 1.
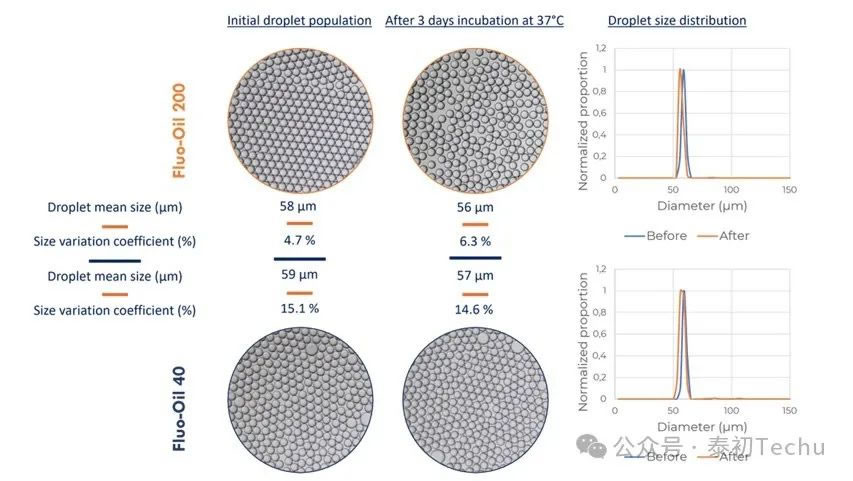
The average droplet diameter of both oils is about 60 μm. Statistical analysis of images shows that FluoSurf? The coefficients of size change (CV) produced by C-stabilized droplets in Fluo-Oil 40 and Fluo-Oil 200 were 15.1% and 4.7%, respectively. After incubation, the droplet sizes of Fluo-Oil 40 and Fluo-Oil 200 were similar, with coefficients of variation (CV) of 14.6% and 6.3%, respectively. The droplets generated in Fluo-Oil 200 have a lower coefficient of variation (CV) in size before and after incubation, meaning that the droplets generated in Fluo-Oil 200 are less dispersed than those generated in Fluo-Oil 40. The difference between the two coefficients before and after incubation for each oil is low, and the stability of the droplets produced throughout the incubation process is constant.
In summary, Fluo-Oil 200 fluoropolymer outperforms Fluo-Oil 40 in terms of droplet dispersion. However, the droplets produced by both oils exhibited excellent stability throughout the incubation process.
III) Dye retention
This section studied the retention of dyes in droplets during the cultivation process using two oils (Fluo-Oil 40 and Fluo-Oil 200). Two dyes were selected: fluorescein and resorcinol.
1. Fluorescein as a model compound
Fluorescein is widely used as a fluorescent dye in microfluidic experiments. In this study, fluorescein was used as a model compound for retention studies.
Two types of oil-in-water droplet microsphere emulsifiers [“empty” (loaded with PBS) and “full” (loaded with 20 μM fluorescein in PBS) droplets] were alternately generated in Fluo-Oil 40 and Fluo-Oil 200 fluorinated oils. The mixture containing both types of droplets (“empty” and “full”) was incubated at 37°C, and photographs were taken at different time points. Images and results of the statistical quantitative analysis of the fluorescence intensity evolution of the full and empty droplets are shown in Figure 2.
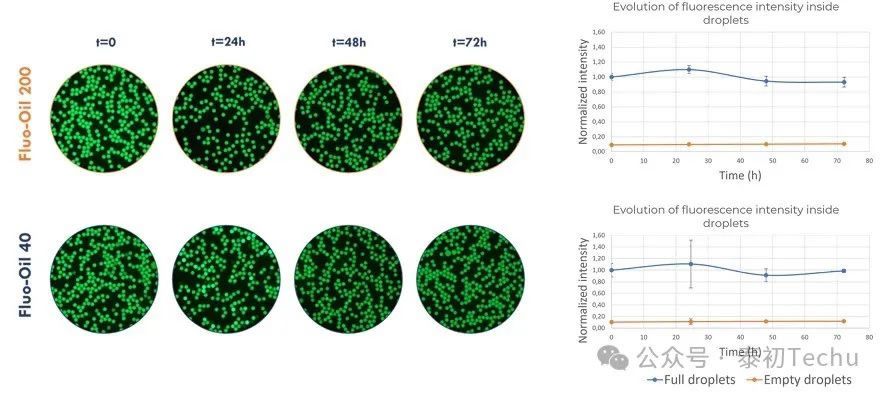
In the image shown in Figure 2, black droplets correspond to empty droplets, while green droplets correspond to droplets containing the fluorescent dye fluorescein. In both oils, no crosstalk was observed between full and empty droplets at t=0 (immediately after formation) and at t=72h (end of incubation). The dye retention rate of fluorescein was excellent in both oils. The assessment of the average fluorescence intensity of full and empty droplets confirmed these results, showing nearly constant values for both oils.
In summary, fluorescein dye retention in droplets produced in Fluo-Oil 200 and Fluo-Oil 40 is highly effective.
2. Reazurin: A common cell viability marker in microbiology
In microbiology, resorcinol is commonly used as a probe for detecting cell viability. In resorcinol-based assays, molecules with low fluorescence intensity are metabolized by cells into resorcinol, which has high fluorescence. Reazurin is such a molecule. Resazurin is blue and has weak fluorescence, but when it comes into contact with living cells, it is reduced to resorcinol, a red, highly fluorescent compound, through a redox reaction, as shown in Figure 3.
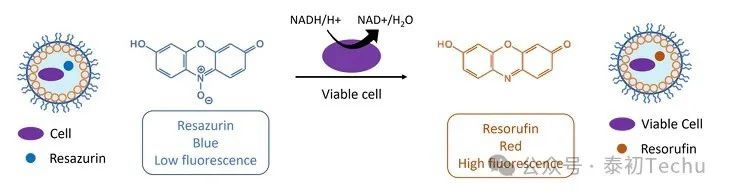
This microbiological test has been applied to the field of microfluidic droplet microfluidics: a cell is encapsulated in a microdroplet using resazurin; if the droplet becomes fluorescent, the cell contained in the fluorescent droplet is viable. For example, this test is suitable for the rapid detection of pathogens in food.
In the first part, the retention of resorcinol in droplets was characterized in two different fluorinated oils. After evaluating the most suitable oil, the concentration of surfactant was optimized. Once the optimal conditions were determined, the retention of resazurin in the droplets and its conversion to resorcinol (containing cysteine) were characterized during the culture process.
● The effect of oil selection on resorufin retention
First, the retention of the fluorescent compound resorufin in the droplets was characterized. As mentioned earlier, two types of oil-in-water droplet emulsion microspheres [“empty” (loaded with PBS) and “full” (loaded with 2 μM resorufin in PBS) droplets] were alternately generated in Fluo-Oil 40 and Fluo-Oil 200, both containing 4% w/w FluoSurf?-C. A mixture of the two droplets (“empty” and “full”) was incubated at room temperature, and photographs were taken at different time points.
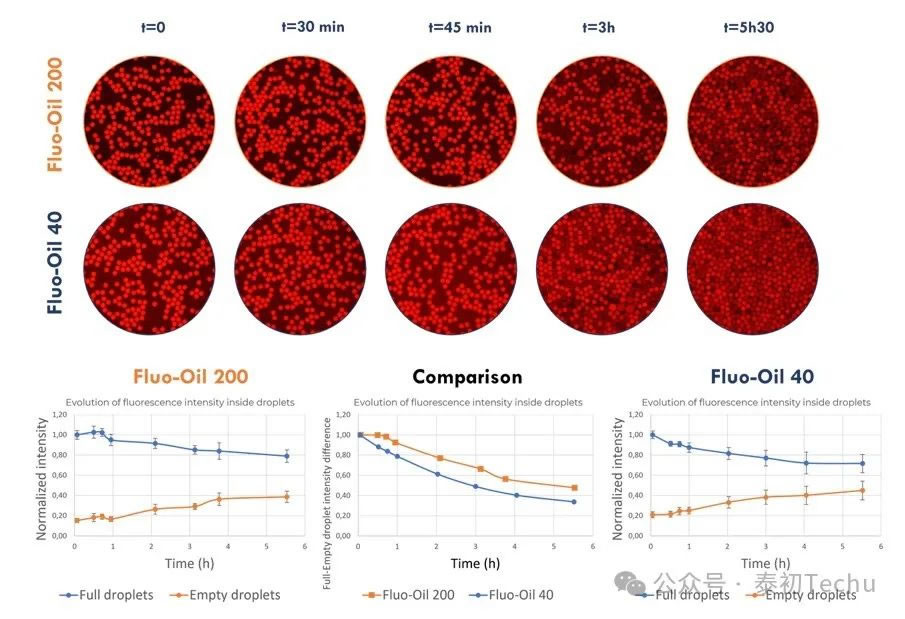
In Figure 4, qualitative and quantitative evaluation of the fluorescence evolution of empty and full droplets generated in Fluo-Oil 200 shows that resorufin begins to leak after 45 minutes of incubation. However, for droplets generated in Fluo-Oil 40, redissolution leakage begins at t=0. Therefore, better resorufin retention is observed in Fluo-Oil 200.
Another method to limit dye leakage in microfluidic experiments is to adjust the concentration of surfactants.
● Effect of surfactant concentration on resorufin retention rate
Two types of oil-in-water emulsions [“empty” (loaded with PBS) and “full” (loaded with 2 μM resorufin in PBS) droplets] were alternately generated in Fluo-Oil 200 containing three different surfactant concentrations. The mixture containing the two droplets (“empty” and “full”) was incubated at room temperature, and photographs were taken at different time points.
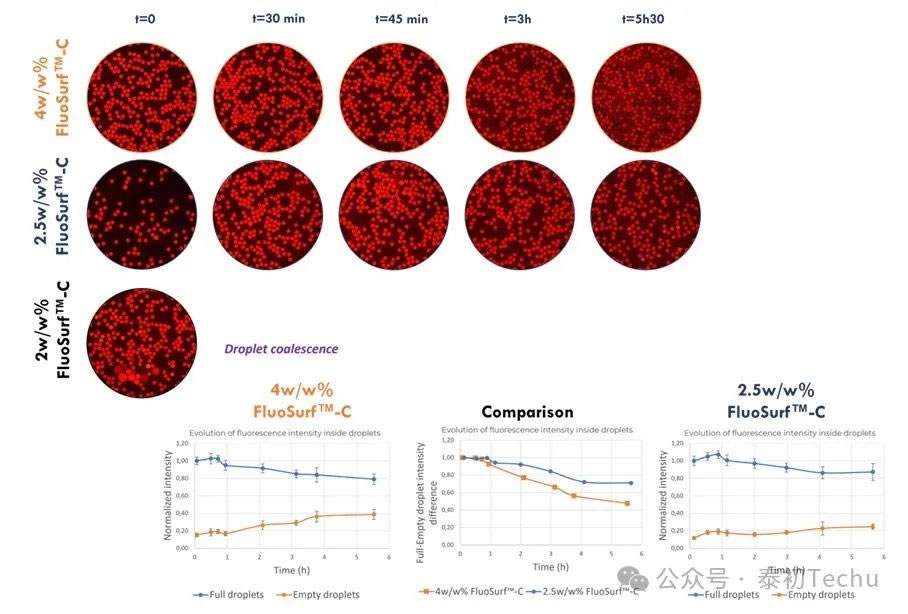
As mentioned earlier, after 45 minutes, resorufin began to leak from the full droplet to the empty droplet in Fluo-Oil 200 containing 4% w/w FluoSurf?-C. When 2% w/w FluoSurf?-C was added to Fluo-Oil 200, the droplets coalesced at t=0. A concentration of 2w/w% is insufficient to ensure the stability of the generated droplets. When using 2.5w/w% FluoSurf?-C in Fluo-Oil 200, resorufin and resorufin-intermediate phenol remain in the droplets for a longer time, and leakage is limited.
Therefore, under our research conditions, the optimal concentration for stabilizing droplets and minimizing dye leakage is 2.5 w/w% FluoSurf?-C in Fluo-Oil 200.
● Determination of cell viability using a resazurin-based cell viability model in droplets generated by Fluo-Oil 200 oil
In this section, model viability was determined using resazurin in droplets. The optimal experimental conditions found in the previous sections (2.5 w/w% FluoSurf?-C in Fluo Oil 200) were used. Cysteine, a compound capable of mimicking the activity of live cells, was also used in the experimental measurements. Droplets containing resazurin (44 μM) in PBS and droplets containing resazurin (44 μM) and cysteine (40 mM) in PBS were alternately generated. The droplets were incubated at room temperature for 4 hours. The experimental protocol is shown in Figure 6.
At the start of the experiment, droplets containing only resazurin are non-fluorescent; during incubation, there is no crosstalk, and the droplets should remain non-fluorescent. On the other hand, at the start of the experiment, droplets containing resazurin and cysteine are non-fluorescent; during incubation, cysteine converts resazurin to resazurin green, and the droplets become fluorescent. The experimental results are shown in Figure 6.
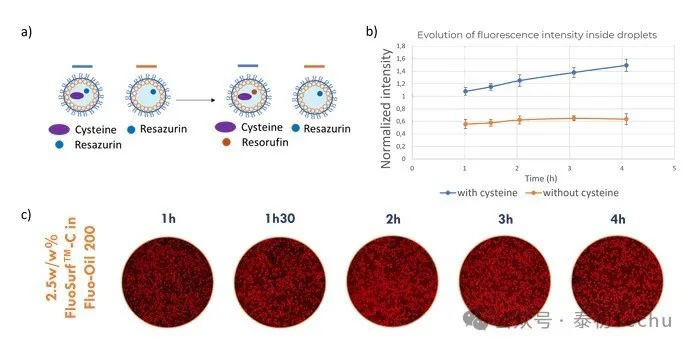
In Figure 6, after 1 hour of incubation, the fluorescence difference between the two droplet populations (droplets containing cysteine and droplets without cysteine) was very small. During incubation, the fluorescence level of the droplets containing cysteine increased because the latter converted resazurin into resorufin. The characterization of fluorescence intensity confirmed this result. In the figure, droplets containing cysteine show a continuous increase in fluorescence intensity. Droplets without cysteine maintain constant fluorescence intensity over 4 hours. The results indicate that there is no crosstalk between droplets over 4 hours. Cell viability experiments suggest an incubation time of 1–4 hours. Here, we have demonstrated that droplet-based microfluidics can be used for resazurin-based cell viability assays by generating droplets under optimal conditions using Fluo-Oil 200 oil.
Conclusion
To improve dye retention in microfluidic experiments, experimental conditions need to be optimized. This includes optimizing surfactant concentration as well as selecting the appropriate fluorinated oil.
In this study, we found that droplet microspheres generated using Fluo-Oil 200 in microfluidic experiments can significantly reduce dye leakage. Therefore, under optimal experimental conditions, the use of Fluo-Oil 200 fluorinated oil enables cell viability assays using resorufin diphenol in droplet-based microfluidics.
Additional information:
FluoSurf Neat surfactant’s biocompatibility and analysis certificates help you effectively understand product characteristics and consistency between multiple products, as well as biocompatibility for biological applications, ensuring you can reuse and repurchase with confidence.