Fluo-Oil 200, abbreviated as FO200, is a biocompatible fluorinated oil that can be used as a replacement for FC40 fluorinated oil / electronic fluorinated fluids. It ensures the stability, biocompatibility, and reproducibility of your microfluidic experiments.
Fluo-Oil 200 is specially formulated for diluting FluoSurf Neat surfactant or diluted FluoSurf surfactant Fluo-Oil.
Fluo-Oil 200 can be stored at room temperature. Currently, packaging options include 100mL/bottle (default), 50mL/bottle, and 30mL/bottle.

The appropriate ratio of fluorinated oils Fluo-Oil 200 and Fluo-Oil 40 (FC40) to surfactants improves the retention of dyes in droplet microspheres.
Droplet microfluidics has emerged as a rapid, precise, quantitative, and cost-effective tool for biological and chemical screening. Monodisperse water-in-oil droplets generated by droplet microfluidic devices carry biological or chemical agents. These droplets microspheres serve as microchambers or reaction compartments. To analyze and/or sort droplet contents, dyes are typically added. Droplets are then monitored and sorted based on their fluorescent signals.
In droplet microfluidic experiments involving dye usage, a common issue is dye leakage. Dye release into the oil phase and adjacent droplets compromises the accuracy of fluorescence monitoring. Parameters influencing dye retention within droplets include: the nature of the buffer or cell culture medium, the choice of fluorophore and its hydrophilicity, and the concentration, properties, and molecular weight of the oil phase and surfactant. The effects of two fluorophores and surfactant concentrations on dye retention rates were discussed. Properties of Fluorinated Oils
In this study, we selected two fluorinated oils: Fluo-Oil 40 and Fluo-Oil 200 to investigate dye leakage in microfluidic experiments. The formulations of both fluorinated oils ensure stability, biocompatibility, and reproducibility in microfluidic experiments as alternatives to Fluorinert FC40 fluorinated oil.
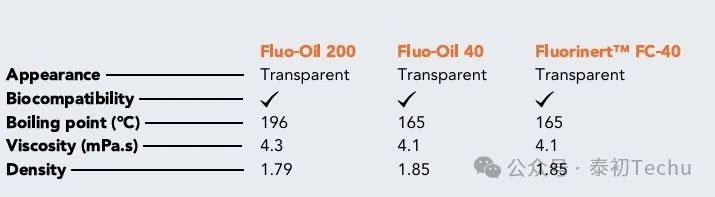
Table 1: Physical properties of Fluo-Oil 200, Fluo-Oil 40, and Fluorinert FC-40
Table 1 presents the physical properties of two fluorinated oils. The performance characteristics of these two fluorinated oils are compared with those of Fluorinert FC-40. The performance of Fluorinert FC-40 is identical to that of Fluo-Oil 40. Fluo-Oil 200 has a slightly lower density than both Fluo-Oil 40 and Fluorinert FC-40, while exhibiting slightly higher boiling points and viscosities. During droplet microsphere formation, minor adjustments to the liquid flow rate may be required to achieve comparable droplets, though the physical properties of both oils remain highly similar. Microfluidic Experiments
To evaluate the impact of fluorinated oil selection on dye leakage, oil-in-water droplet microspheres were generated using both oils. Evaluation parameters included droplet size distribution, stability after incubation at 37°C, and molecular retention.
The PDMS/glass microfluidic chip used for droplet microsphere generation featured a flow-focusing geometry. Microchannels were treated with the hydrophobic reagent Fluo-ST2 (hydrophobic surface treatment). In studies comparing the two fluorinated oils, the oil continuous phase was prepared by dissolving 4w/w% pure FluoSurf-C surfactant in either Fluo-Oil 200 or Fluo-Oil 40. The dispersed aqueous phases used in these experiments were PBS, 20 μM fluorescein PBS, 2 μM resorufin PBS, or 44 μM resazurin + 40 mM cysteine PBS. The two fluorophores were compared by analyzing mixtures of empty droplets and droplets containing fluorescent dyes. Droplet stability experiments were conducted using an injection pump to control the flow rates of different phases in the microfluidic droplet chip (oil phase: 300 μL/h, aqueous phase: 100 μL/h); dye retention experiments were performed at oil phase: 600 μL/h, aqueous phase: 100 μL/h.
Droplet formation was monitored using an inverted microscope. Image analysis was performed using ImageJ software. II) Droplet Stability After Incubation Droplet stability was investigated through incubation in two fluorophore oils (Fluo-Oil 40 and Fluo-Oil 200). The size distribution of droplets was analyzed before and after 3 days of incubation at 37°C.
The results are shown in Figure 1.
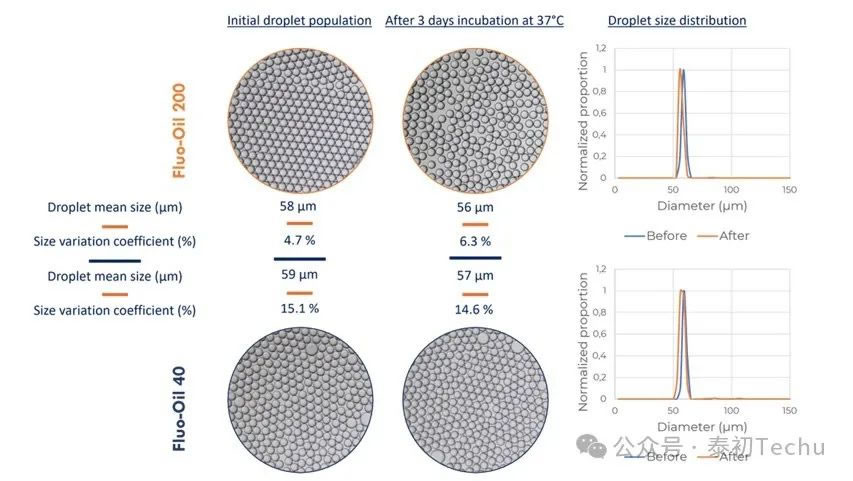
Figure 1: Size distribution of water-in-oil droplets and corresponding photographs for Fluo-Oil 200 and Fluo-Oil 40 before and after incubation at 37°C for 3 days.
The average droplet diameter for both oils was approximately 60μm. Statistical analysis of the images revealed that the coefficient of variation (CV) for droplet size variation in Fluo-Surf-C-stabilized droplets was 15.1% in Fluo-Oil 40 and 4.7% in Fluo-Oil 200. After incubation, droplets in Fluo-Oil 40 and Fluo-Oil 200 exhibited similar sizes, with coefficients of variation (CV) of 14.6% and 6.3%, respectively. Droplets generated in Fluo-Oil 200 exhibited lower CV values both before and after incubation, indicating less dispersion compared to those in Fluo-Oil 40. The difference between the two coefficients for each oil was minimal, demonstrating consistent droplet stability throughout the incubation process.
In summary, Fluo-Oil 200 demonstrated superior droplet dispersion compared to Fluo-Oil 40. However, droplets from both oils exhibited excellent stability throughout the incubation process.
III) Dye Retention
This section investigates dye retention within droplets during incubation for both oils (Fluo-Oil 40 and Fluo-Oil 200). Two dyes were selected: fluorescein and resorufin.
1. Fluorescein as a Model Compound
Fluorescein, a widely used fluorescent dye in microfluidic experiments, served as a model compound for retention studies in this research.
Two types of oil-in-water droplet microsphere emulsifiers [“empty” (loaded with PBS) and “full” (loaded with 20 μM fluorescein in PBS) droplets) were alternately generated in Fluo-Oil 40 and Fluo-Oil 200 fluorophore oils. The mixture containing both droplets (“empty” and “full”) was incubated at 37°C, with images captured at various time points. Images and results from statistical quantitative analysis of the fluorescence intensity evolution in full and empty droplets are shown in Figure 2.
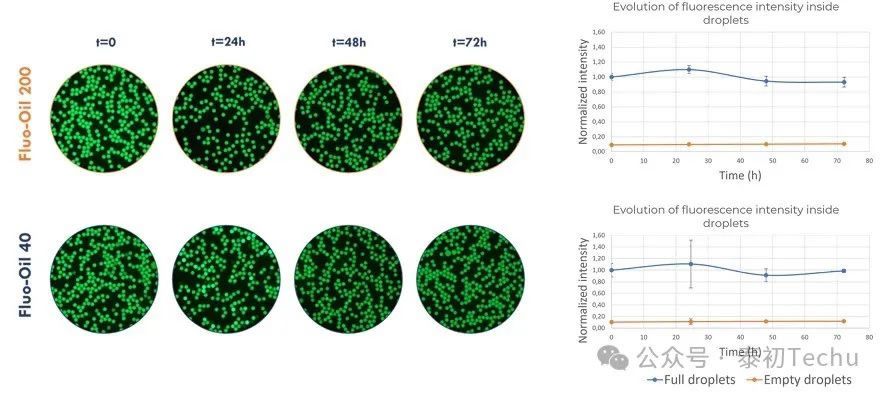
Figure 2: Images of full and empty droplets mixed in Fluo-Oil 200 and Fluo-Oil 40 during incubation at 37°C, and the evolution of average fluorescence intensity.
In the image shown in Figure 2, black droplets correspond to empty droplets, while green droplets correspond to droplets containing the fluorescent dye fluorescein. In both oils, no crosstalk between full and empty droplets was observed at t=0 (immediately after generation) or at t=72h (end of incubation). Fluorescein dye retention was excellent in both oils. Evaluation of the average fluorescence intensity in full and empty droplets confirmed these results, showing nearly constant values for both oils.
To summarize, the retention of fluorescein dye in droplets generated from Fluo-Oil 200 and Fluo-Oil 40 is highly effective.
2. Resorufin: A Common Cell Viability Marker in Microbiology
In microbiology, resorufin is frequently used as a probe for detecting cell viability. In resorufin-based assays, molecules with low fluorescence intensity are metabolically converted by cells into highly fluorescent resorufin. Resazurin is one such molecule. Resazurin appears blue with weak fluorescence, but upon contact with living cells, it undergoes a redox reaction and is reduced to resorcinol, a red, highly fluorescent compound, as shown in Figure 3.
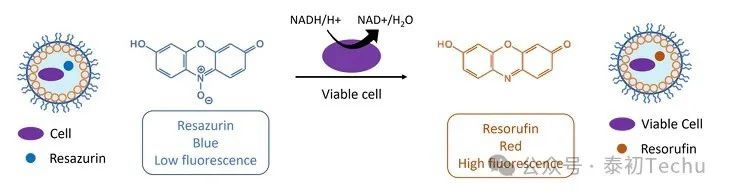
Figure 3: The scheme shows how resorufin is used as a fluorescent probe to test cell viability.
This microbiological assay has been applied in the field of microfluidic droplet-based systems: a cell is encapsulated within a droplet containing resazurin; if the droplet fluoresces, the cell within the fluorescent droplet is viable. For instance, this assay is suitable for rapid detection of pathogens in food. Part 1 characterized the retention of resazurin within droplets in two different fluorinated oils. After evaluating the most suitable oil, the surfactant concentration was optimized. Once optimal conditions were established, the retention of resazurin within droplets and its conversion to resorufin (containing cysteine) were characterized during the culture process.
● Effect of oil selection on resorufin retention
First, the retention of the fluorescent compound resorufin in droplets was characterized. As previously described, two types of oil-in-water droplet emulsion microspheres [“empty” droplets (loaded with PBS) and “full” droplets (loaded with 2 μM resorufin in PBS)] were alternately generated in Fluo-Oil 40 and Fluo-Oil 200 containing 4% w/w FluoSurf?-C. The mixture containing both droplets (“empty” and “full”) was incubated at room temperature, and images were captured at different time points.
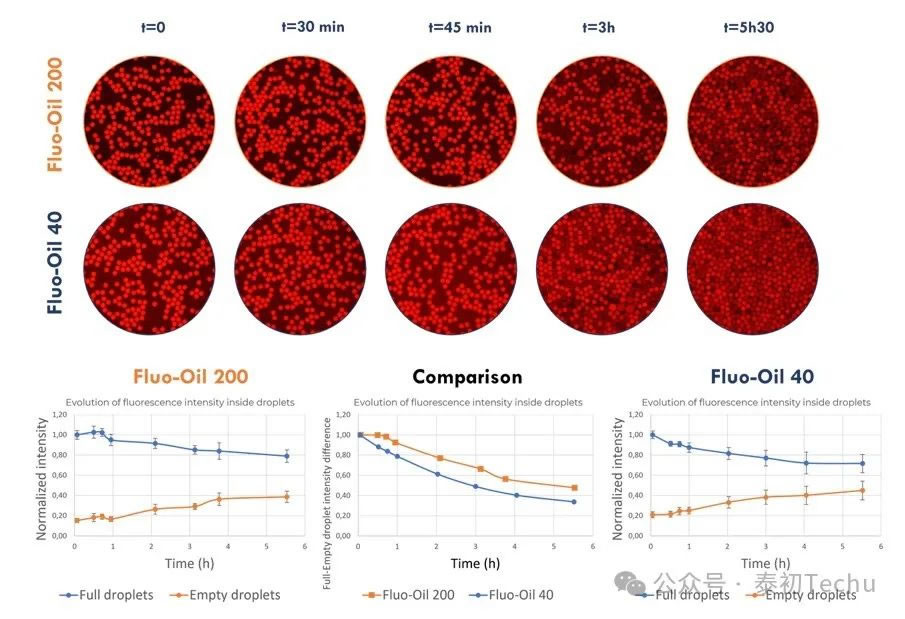
Figure 4: Evolution of full-drop and empty-drop mixing and average fluorescence intensity in oil-in-water droplets of Fluo-Oil 200 and Fluo-Oil 40 during incubation at room temperature.
In Figure 4, qualitative and quantitative evaluation of the fluorescence evolution in empty and full droplets generated in Fluo-Oil 200 shows that resorufin leakage begins after 45 minutes of incubation. However, for droplets generated in Fluo-Oil 40, redissolution leakage commenced at t=0. Consequently, superior resorufin retention was observed in Fluo-Oil 200. Another method to limit dye leakage in microfluidic experiments is adjusting surfactant concentration. ● Effect of surfactant concentration on resorufin retention. (loaded with 2 μM resorufin in PBS) droplets]. The mixture containing both droplets (“empty” and “full”) was incubated at room temperature, and photographs were taken at different time points.
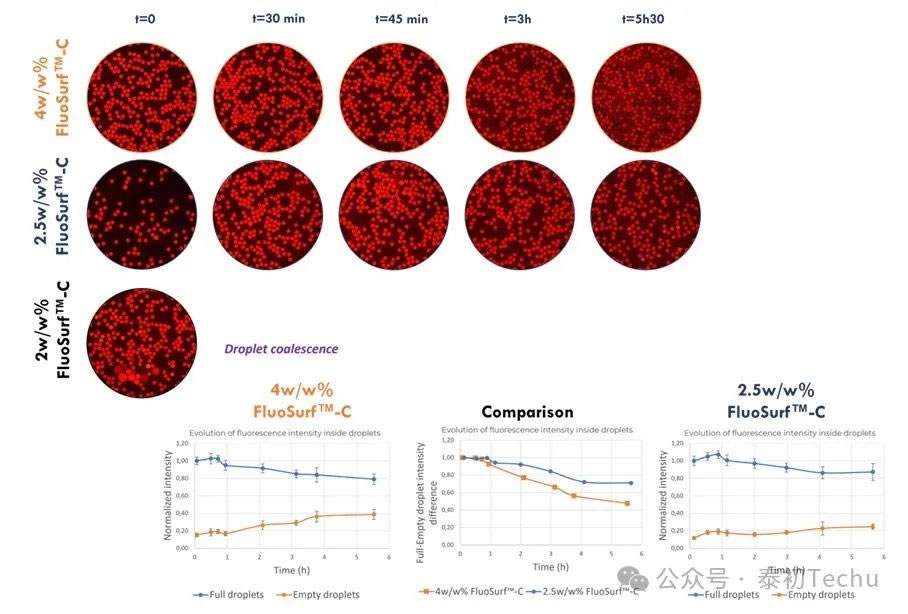
Figure 5: Evolution of mixing and average fluorescence intensity of oil-in-water droplet microspheres generated using 4 w/w%, 2.5 w/w%, and 2 w/w% FluoSurf-C in Fluo-Oil 200 at room temperature.
As previously described, after 45 minutes, resorufin began leaking from the full droplet to the empty droplet in Fluo-Oil 200 containing 4% w/w FluoSurf-C. When 2% w/w FluoSurf-C was added to Fluo-Oil 200, the droplets coalesced and merged at t=0. The 2% w/w concentration was insufficient to ensure the stability of the generated droplets. Using 2.5w/w% FluoSurf-C in Fluo-Oil 200, resorufin and resorufin-meso-phenol remained in the droplets longer, with leakage being limited.
Therefore, under our experimental conditions, the optimal concentration for stabilizing droplets and minimizing dye leakage is 2.5% w/w FluoSurf-C in Fluo-Oil 200.
● Resazurin-based cell viability model in droplets generated by Fluo-Oil 200 oil
In this section, a model viability assay using resazurin was performed in droplets. The optimal experimental conditions identified in previous sections (2.5 w/w% FluoSurf-C in Fluo-Oil 200) were employed. Cysteine, a compound capable of mimicking live cell activity, was also incorporated into the assay. Droplets containing resazurin (44 μM) in PBS and droplets containing resazurin (44 μM) and cysteine (40 mM) in PBS were alternately prepared. Droplets were incubated at room temperature for 4 hours. The experimental setup is illustrated in Figure 6.
At the start of the experiment, droplets containing only resazurin were non-fluorescent; during incubation, no crosstalk occurred, and the droplets should remain non-fluorescent. Conversely, droplets containing both resazurin and cysteine were non-fluorescent at the start; during incubation, cysteine converted resazurin from blue to green, causing the droplets to fluoresce. Experimental results are shown in Figure 6.
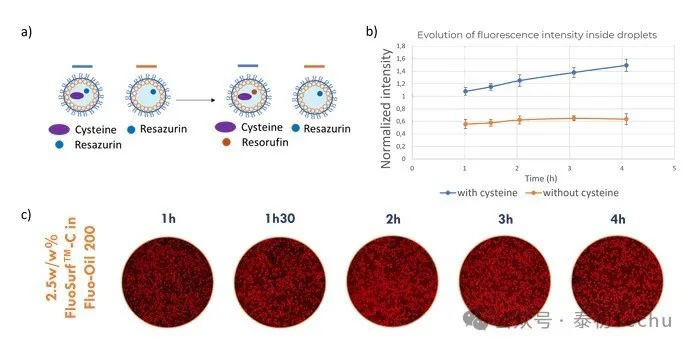
Figure 6. a) Experimental setup: Reazurin is converted to reazurin only in droplets containing cysteine. b) Evolution of average fluorescence intensity during incubation of oil-in-water droplets containing cysteine and without cysteine in Fluo-Oil 200. c) Photographs of mixed full and empty droplets throughout the incubation process.
In Figure 6, after 1 hour of incubation, the fluorescence difference between the two droplet populations (droplets containing cysteine and droplets without cysteine) was very small. During incubation, the fluorescence level of the droplets containing cysteine increased because the latter converted resazurin into resorufin. The characterization of fluorescence intensity confirmed this result. In the figure, droplets containing cysteine show a continuous increase in fluorescence intensity. Droplets without cysteine maintain constant fluorescence intensity over 4 hours. The results indicate that there is no crosstalk between droplets over 4 hours. Cell viability experiments suggest an incubation time of 1–4 hours. Here, we have demonstrated that droplet-based microfluidics can be used for resazurin-based cell viability assays by generating droplets under optimal conditions using Fluo-Oil 200 oil.
Conclusion
To improve dye retention in microfluidic experiments, experimental conditions need to be optimized. This includes optimizing surfactant concentration as well as selecting the appropriate fluorinated oil.
In this study, we found that droplet microspheres generated using Fluo-Oil 200 in microfluidic experiments can significantly reduce dye leakage. Therefore, under optimal experimental conditions, the use of Fluo-Oil 200 fluorinated oil enables cell viability assays using resorufin in droplet-based microfluidics.
Additional information:
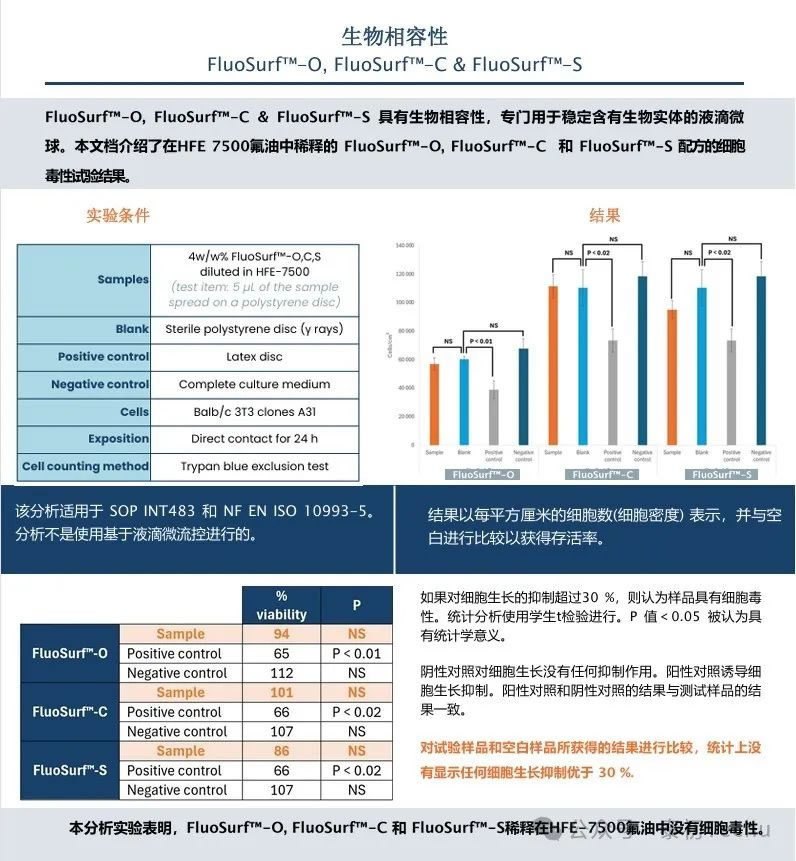
FluoSurf Neat surfactant’s biocompatibility and analysis certificates help you to effectively understand product characteristics and consistency between multiple products, as well as biocompatibility for biological applications, ensuring you can reuse and repurchase with confidence.